LTS and AWAKN LIFE SCIENCES enter into global licensing agreement for proprietary sublingual (S)-ketamine formulation
Andernach – LTS Lohmann Therapie-Systeme AG (“LTS”), a leading pharmaceutical technology company and Awakn Life Sciences Corp. (NEO: AWKN, OTCQB: AWKNF, FSE: 954) (“Awakn”), a clinical-stage biotechnology company developing therapeutics to treat addiction with a near-term focus on Alcohol Use Disorder (AUD), announced today that they have signed a global licensing agreement. The agreement is for a proprietary (S)-ketamine formulation, administered sub-lingually via an oral thin film (OTF) and Awakn will have global exclusivity of its use for Treatment of Addiction, Anxiety Disorders, and Eating Disorders.
LTS has successfully completed a Phase I clinical trial and filed patents in the US and key international markets of China, Canada, Europe, and Japan for this novel formulation of (S)-ketamine. Under the terms of the agreement Awakn has secured exclusive global rights to this Phase I data and the proprietary formulation for use in the above indications. Thereby ensuring strong intellectual property protection and potential to rapidly progress to late clinical stage trials.
The sublingual (S)-ketamine formulation signifies a remarkable potential advancement in addiction treatment. Its sublingual administration offers potentially a faster onset of action, more precise dosing, and reduced potential for adverse effects compared to conventional delivery methods. Safety, efficacy, and patient well-being are paramount in Awakn and LTS’s commitment to transforming the treatment of addiction.
The licensing agreement includes terms related to milestone payments, royalties and to commercial manufacturing of the drug product at LTS.
Commenting on this agreement, Anthony Tennyson, CEO of Awakn, stated, “This exclusive global licensing agreement represents a significant milestone in our mission to provide innovative and effective treatments for addiction, while also providing scope for other mental health disorders. We are confident that LTS pioneering sublingual (S)-ketamine formulation will be a game-changer for those suffering from these conditions. We are excited to lead the way in this mental healthcare revolution.”
“At LTS, we are unrelenting in our commitment to make life better for patients,” commented Bas van Buijtenen, CEO of LTS. “Under this agreement, we will deploy our full expertise and experience to the development and commercial manufacturing of this new therapeutic option in an area that fits perfectly with our strategic focus. The cooperation with Awakn is an opportunity to demonstrate once again how LTS creates commercial value at every stage: from product development to launch.”
About Awakn Life Sciences Corp.
Awakn Life Sciences Corp. is a clinical-stage biotechnology company developing therapeutics targeting addiction. Awakn has a near-term focus on Alcohol Use Disorder (AUD), a condition affecting approximately 51 million people in the US and key European international markets and 285m people globally for which the current standard of care is inadequate. Our goal is to provide breakthrough therapeutics to addiction sufferers in desperate need and our strategy is focused on commercializing our R&D pipeline across multiple channels.
www.AwaknLifeSciences.com
About LTS
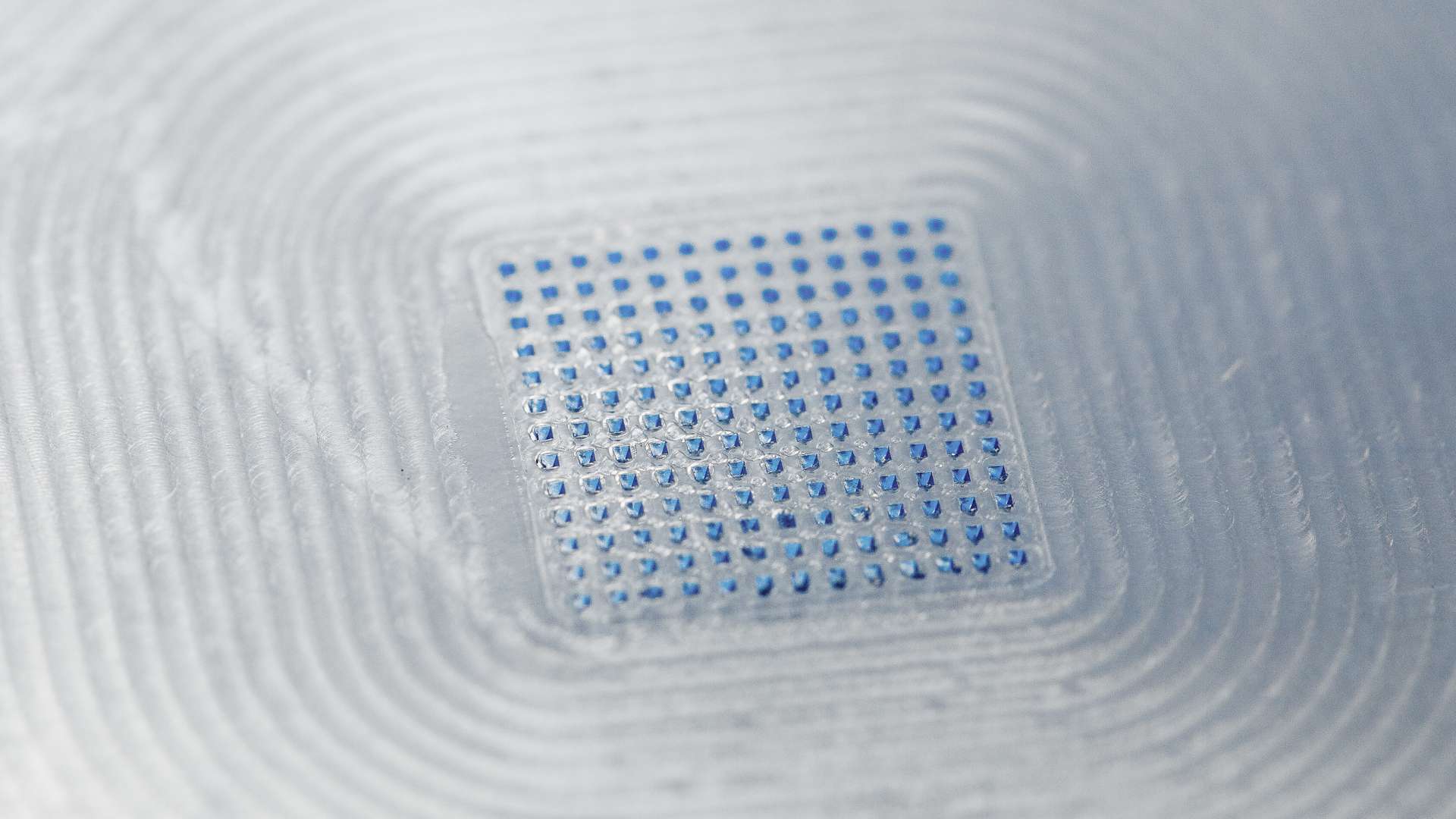
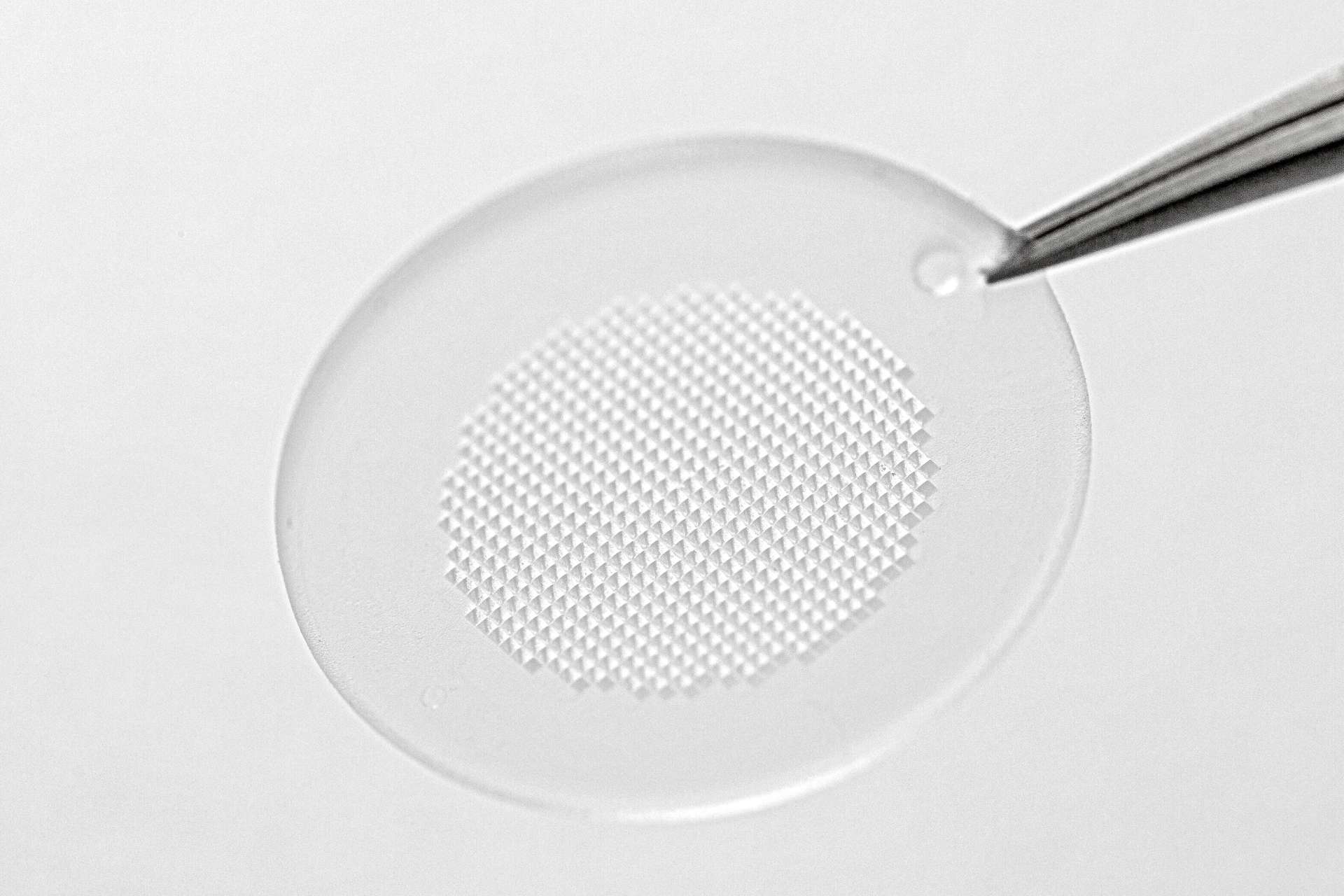
We CARE. We CREATE. We DELIVER. The driving philosophy behind LTS. As a trusted technology partner for the pharmaceutical industry, we develop and manufacture innovative drug delivery systems such as Transdermal Patches (“TTS”) and Oral Thin Films (“OTF”) as well as wearable drug delivery devices (“OBDS”). LTS´ commercial offering encompasses more than 20 marketed products and a diverse pipeline of more than 40 development projects targeting multiple disease indications. LTS’s innovation pipeline contains both partner-funded as well as proprietary, LTS-funded projects. LTS maintains its leading position through the continuous refinement of its core TTS and OTF technologies and by advancing emerging drug delivery technologies, including Microneedle Array Patches (“MAP”) for the transdermal delivery of small and large molecules, biological actives and vaccines. With its SorrelTM wearable drug delivery platform LTS offers patient friendly solutions for complex drugs delivery at home. Founded in 1984, LTS operates today from four sites: in Andernach, Germany, West Caldwell, NJ, USA, St. Paul, MN, USA and Netanya, Israel. LTS has also a representative office in Shanghai, China.
Contact
Dr Iris Schnitzler
iris.schnitzler@ltslohmann.com