EsoCap and LTS announce major milestone achievement in their cooperation
Andenach, 25.06.2021
LTS Lohmann Therapy-Systeme AG (LTS) and EsoCap AG are pleased to announce completion of manufacture of the CTS (Clinical Trial Samples) for the ACESO clinical study conducted by EsoCap as a major milestone in their cooperation on industrial development of a unique drug delivery technology for diseases of the upper gastrointestinal tract. ACESO is a randomized, placebo-controlled, double-blind trial evaluating the efficacy, tolerability and safety of ESO-101 in adult patients with active EoE (eosinophilic esophagitis). The ACESO study is being conducted in 14 centers in Switzerland, Germany, the Netherlands and Spain.
LTS was in charge of GMP (Good Manufacturing Practice) production of CTS, consisting of a thin film loaded with the active compound and rolled up in a capsule. The capsule was assembled in a specially developed mouthpiece and individually packed in a pouch.
“We are particularly pleased to leverage the expertise of a strong partner in the thin film industry”, said Isabelle Racamier, EsoCap CEO. “Our technology offers maximum flexibility as multiple relevant drug substances, including biologics and further innovative compounds, can be incorporated into the film, making our drug delivery platform applicable to various esophageal diseases. “
“As a company committed to delivering pharmaceutical solutions based on our thin film technology, we are greatly interested in moving through the clinical development stages towards commercialisation”, said Dr. Hanshermann Franke, Senior VP Research and Development at LTS. “We are thrilled by the potential for multiple applications for EsoCap’s highly innovative technology in a broad range of esophageal diseases.”
About LTS Lohmann Therapie-Systeme AG
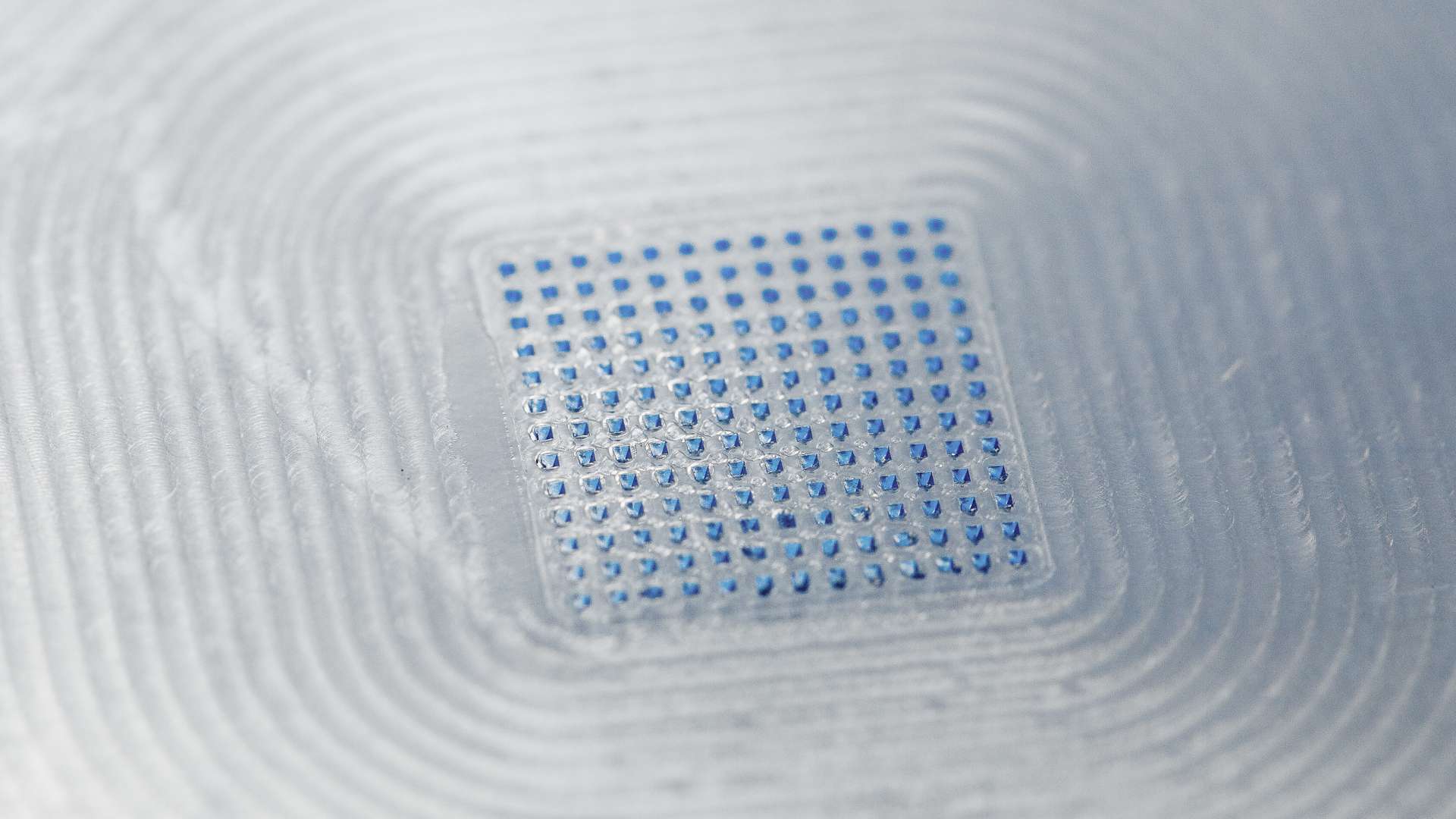
LTS is a leading pharmaceutical technology company that develops and manufactures innovative drug delivery systems, such as Transdermal Patches (“TTS”) and Oral Thin Films (“OTF”), for the pharmaceutical industry. LTS´s commercial offering encompasses more than 20 marketed products and a diverse pipeline of more than 30 development projects targeting multiple disease indications. LTS’s innovation pipeline contains both partner-funded and proprietary, LTS-funded projects. LTS maintains its leading position through continuous refinement of its core TTS and OTF technologies and by advancing emerging drug delivery technologies, including Micro Array Patches for transdermal delivery of large molecule, biological actives. Founded in 1984, LTS operates today from two sites in Andernach, Germany and West Caldwell, New Jersey, USA, with a representation in Shanghai, China.
Contact:
Dr. Iris Schnitzler, E-Mail: iris.schnitzler@ltslohmann.com
About EsoCap AG
EsoCap AG is a Swiss privately funded company based in Basle, Switzerland.
EsoCap’s vision is to improve the lives of patients with serious diseases through development of a unique topical drug delivery platform for diseases of the upper gastrointestinal tract.
Topical treatment in the upper gastrointestinal tract is extremely difficult to achieve due to ultra-short transit times, with less than two seconds from the mouth to the stomach.
EsoCap owns a unique drug delivery platform, allowing the topical application of drug substances for local treatment of diseases of the upper gastrointestinal tract. For more information, please visit www.esocapbiotech.com.
Contact:
EsoCap AG
Malzgasse 9
4052 Basel
Switzerland
isabelle.racamier@esocapbiotech.com