LTS Inaugurates a New Semi-automated Commercial Production Line and Announces Global Manufacturing to Support SorrelTM Wearable Device Market Expansion
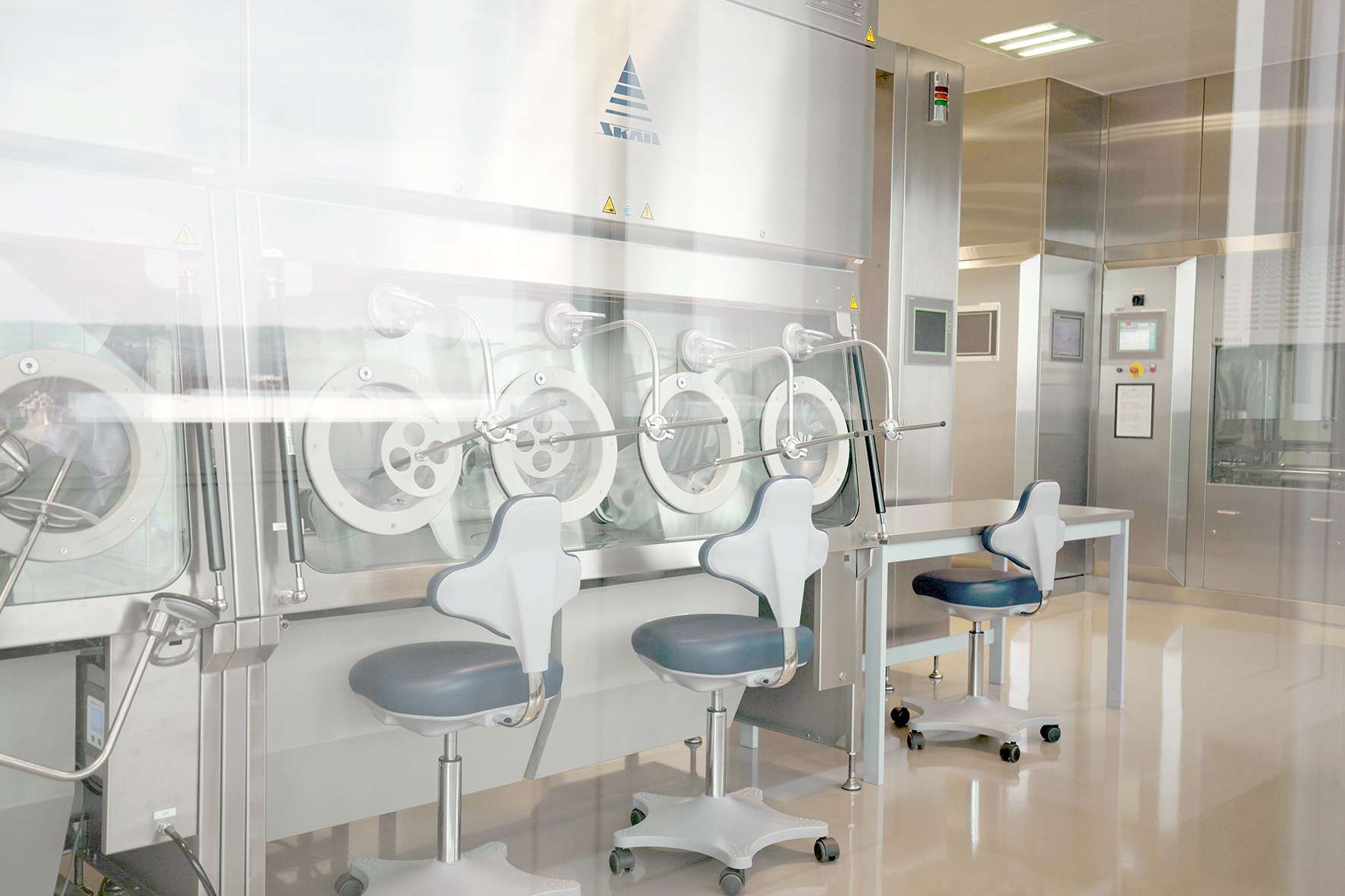
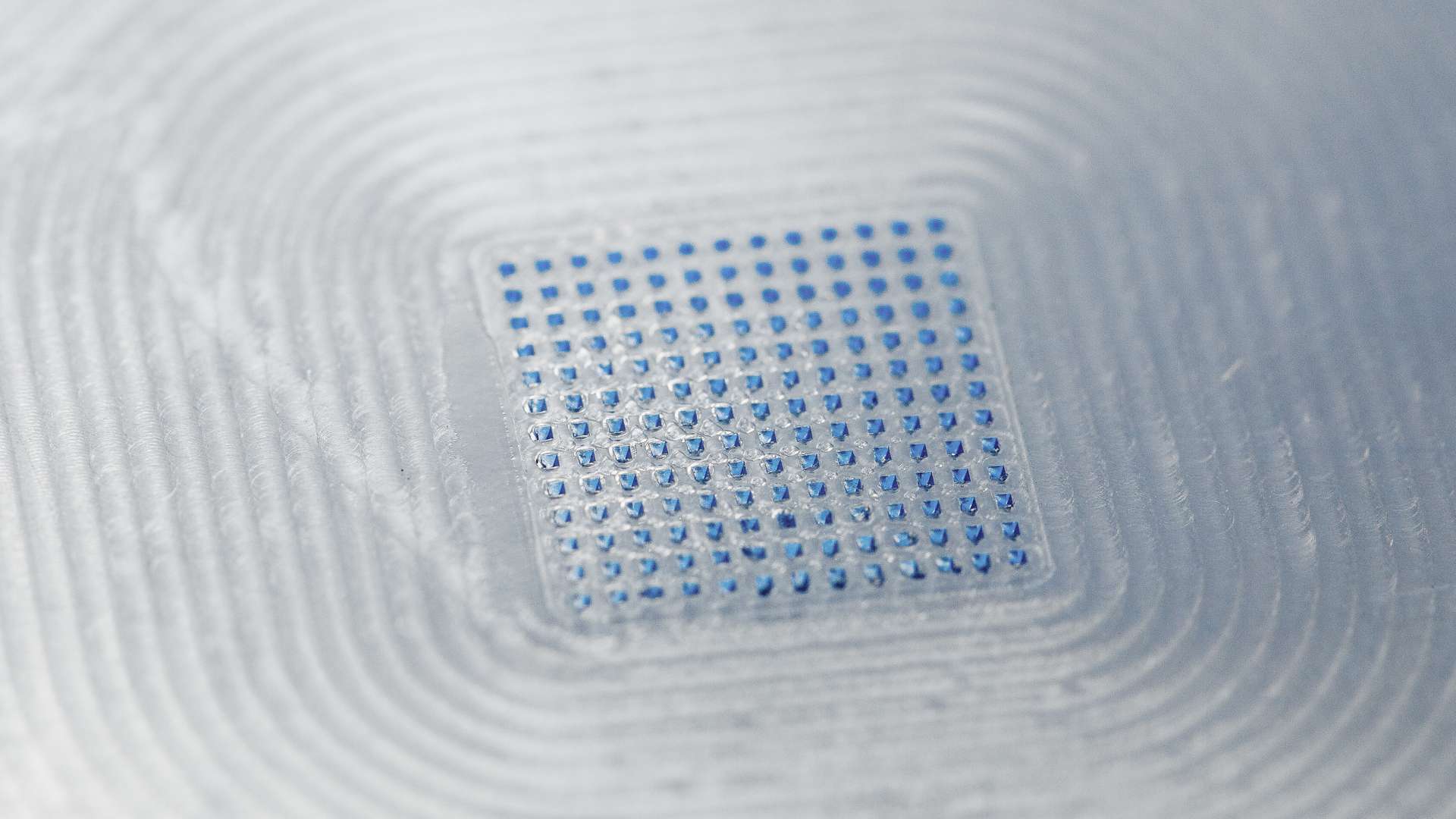
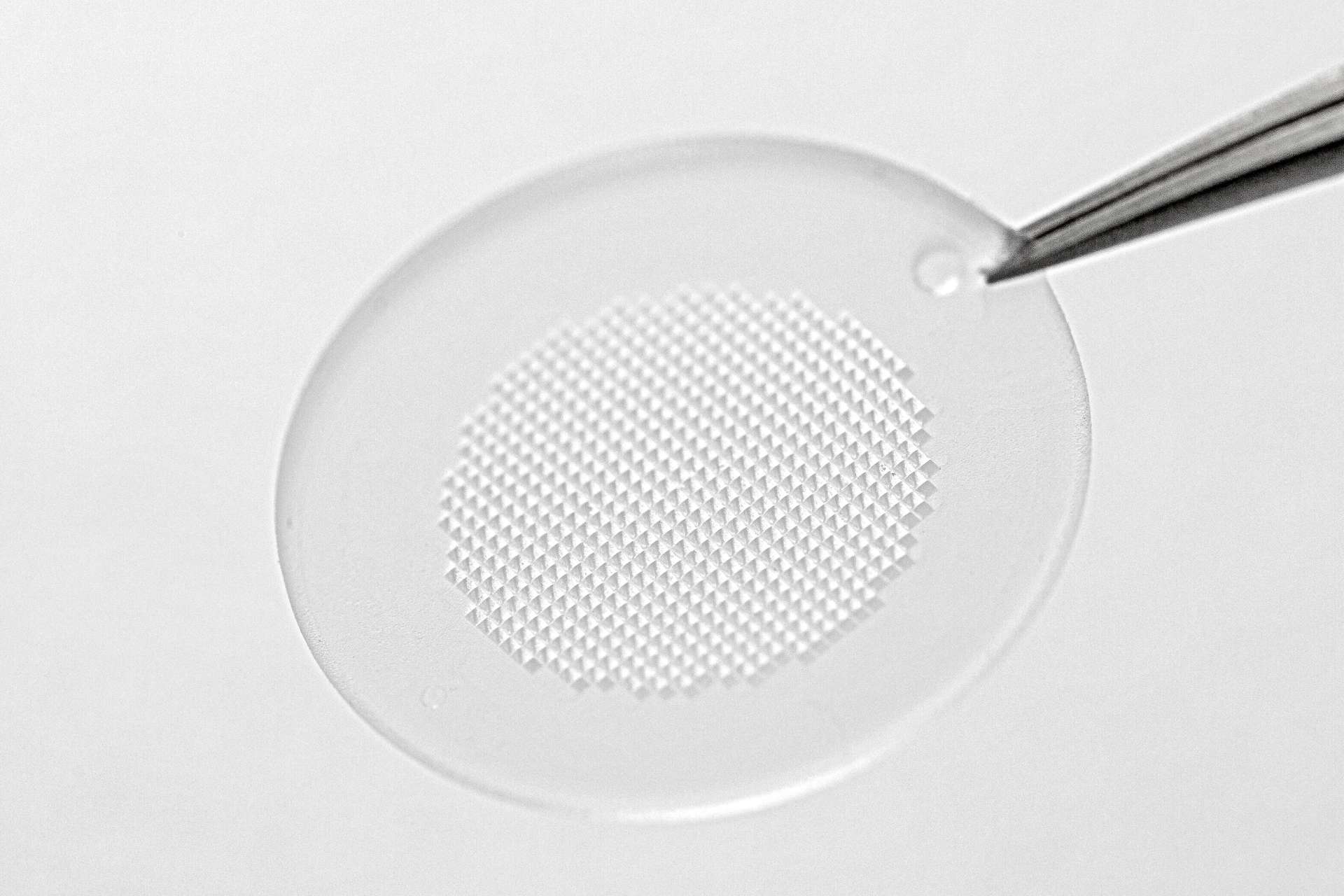
LTS today announced the successful commercial validation of a semi-automated production line for the Sorrel™ wearable drug delivery platform.