Wie wir die vermeintlichen Herausforderungen rund um MAPs annehmen.
Over the years, the advantages of intradermal drug delivery have been well documented.
However, the use of injectables usually requires a relatively sophisticated cold chain, and aversion to needles, pain and needle size are all very real concerns for many patients. In addition, injections often require the time and effort of a healthcare professional (HCP), who will then be exposed to the risk of needlestick injuries (NSIs), with the World Health Organization (WHO) noting that two million HCPs experience percutaneous exposure to infectious diseases each year.
Transdermal patches continue to present an alternative delivery mechanism that addresses many of these challenges. Today, fewer than 30 APIs have been successfully commercialized for Transdermal Therapeutic Systems (TTS) as the choice of API remains relatively limited to small molecules.
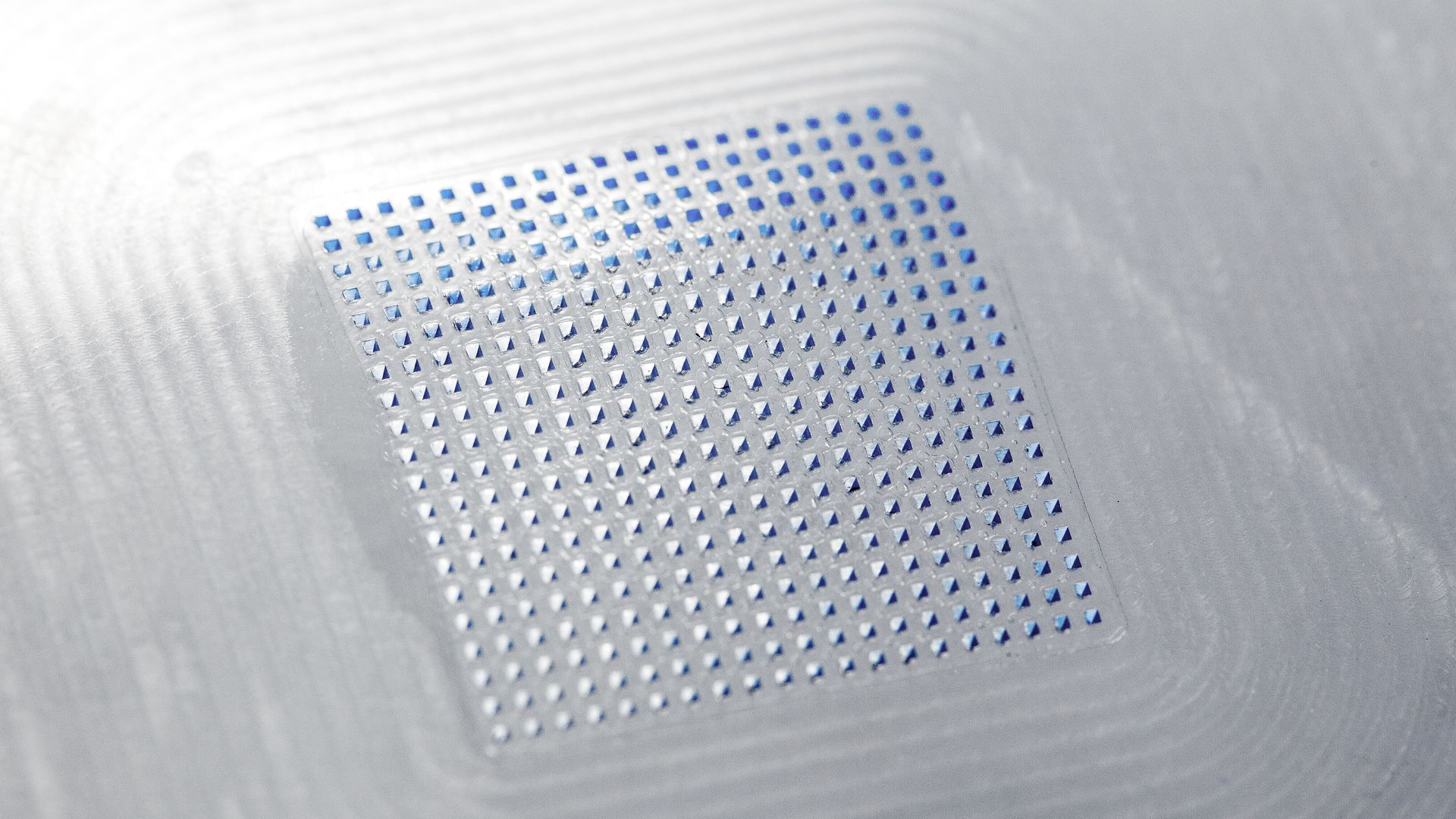
A further answer can be found in microneedle technologies, such as the microarray patches (MAPs) developed by LTS, which can reproducibly deliver APIs into the dermal and epidermal layers of the skin containing high densities of immune cells. This technology also provides an answer to the particular challenges related to injectables in areas of patient compliance, usability and safety for HCPs.
First conceptualized and subsequently patented in the 1950s, the benefits of microneedle technology took some time to be fully recognised, with a paper in 1998 exploring the possibility of using microneedles for vaccination. Since then, the World Health Organisation (WHO) has identified microneedles as a potential game-changer for vaccine distribution and coverage, especially in low-to-middle income countries.
Variations on microneedle technology include solid removable microneedles, coated microneedles and hollow microneedles, albeit these options still present some sharps challenge upon removal for waste. This issue is eradicated in the case of dissolvable microneedles and hydrogel-forming microneedles.
Despite the benefits of these microneedle innovations in terms of patient compliance and HCP safety, the FDA’s experience of submissions relating to microneedles to date could, perhaps be described as disappointing, with quite basic failures evident in many fundamental aspects of submission.
In response, in October 2020 the FDA set out clear criteria for a successful submission. It outlined the need to deliver: a comprehensive design control package that identifies risks and hazards; a clearly defined control strategy for enhanced permeation and retention (EPR), design verification and validation; a risk management plan; and an iterative guide to risk management activities throughout the entire product lifecycle.
In our opinion, the requirements set out by the FDA are nothing more than would be expected for any regulated drug delivery device, and it is alarming that the quality of some submissions has necessitated such regulatory guidance, given the immediate and unparalleled opportunities this kind of technology presents.
For 40 years, LTS has been a trusted partner and market leader in innovative transdermal drug delivery systems. Our mission is to find alternatives for patients where conventional drug delivery causes challenges. We believe that if the API can be delivered in an injection, it can probably be delivered in a MAP. We also believe that the clear potential in MAPs, which has been untapped to date, is now ready to be realised using the LTS technology platform.
For pharma partners, there is now the necessary evidence from LTS to demonstrate that MAPs are a safe and reliable dosage form and adequate patient dosing can be achieved, making the technology a strong option for repurposing existing products into a more patient-friendly delivery mechanism. There is also a very clear opportunity to develop new products, especially in the areas of biologicals, vaccines and difficult to deliver small molecules.
For payers, there is a real benefit to MAPs in that they can enable patient self-administration, creating the potential for lower healthcare costs. For HCPs, they help to reduce the challenges and threats associated with needlestick injuries. And for the patient, the reduced pain and lower psychological challenge of administering MAP technologies creates the potential for increased compliance and adherence.







